RESEARCH
mRNA Cancer Advance Hints at New Path for In-Body Therapeutics
BNT142 shows early clinical activity as mRNA oncology platforms gain momentum
5 Dec 2025
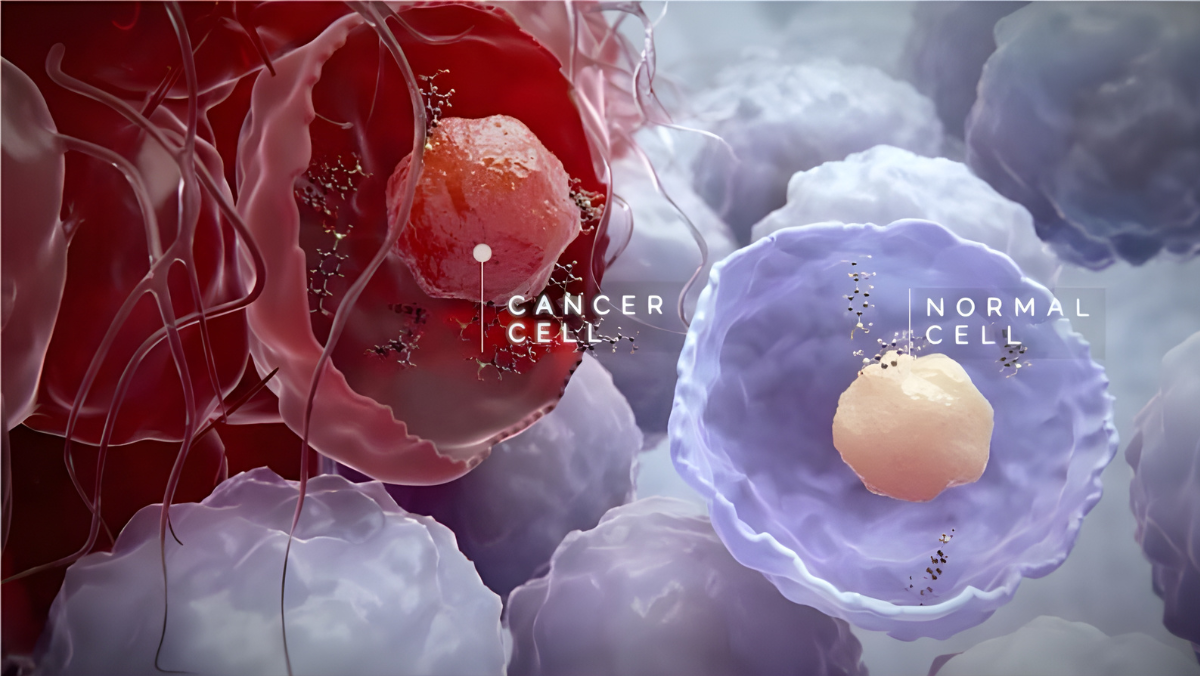
BioNTech’s experimental mRNA therapy BNT142 has shown early signs of clinical activity and a manageable safety profile in Phase I and II studies, prompting quiet interest across the biotechnology sector as companies test whether the technology can extend beyond vaccines.
The treatment instructs the body to produce its own cancer-targeting molecule rather than rely on laboratory-made antibodies. Initial updates point to disease stabilisation in patients with advanced tumours, while safety findings so far are consistent with a first-in-human study. Investigators stressed that the data are preliminary and that “real judgments lie many months ahead”.
The programme reflects a wider push by drugmakers to adapt mRNA platforms for oncology, rare diseases and immune disorders. BioNTech and Moderna have expanded research agreements with partners including Merck, CytomX, OncoC4 and DualityBio, part of a broader effort to test whether in-body manufacturing can complement or simplify traditional biologic development.
Regulators are also considering how to evaluate mRNA therapies that do not fit neatly within existing categories. Questions about durability of response and long-term safety remain central and are likely to influence development timelines as much as any upcoming dataset.
Further trial readouts for BNT142 and other mRNA candidates are expected over the next year. If early signals hold, mRNA platforms could take a more defined place in oncology’s evolving treatment toolkit.
Latest News
18 Feb 2026
How the Cloud Powers the Next mRNA Breakthrough16 Feb 2026
Can Europe Win the mRNA Cancer Race?12 Feb 2026
FDA Refusal-to-File Resets mRNA Flu Strategy11 Feb 2026
Britain’s Bet On Better RNA Factories
Related News

TECHNOLOGY
18 Feb 2026
How the Cloud Powers the Next mRNA Breakthrough
INSIGHTS
16 Feb 2026
Can Europe Win the mRNA Cancer Race?
REGULATORY
12 Feb 2026
FDA Refusal-to-File Resets mRNA Flu Strategy
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.