REGULATORY
US MRNA Leaders Push for Regulatory Modernization as Innovation Surges
Moderna, Replicate, and others accelerate mRNA advances as the US faces rising pressure to update its regulatory approach
20 Nov 2025
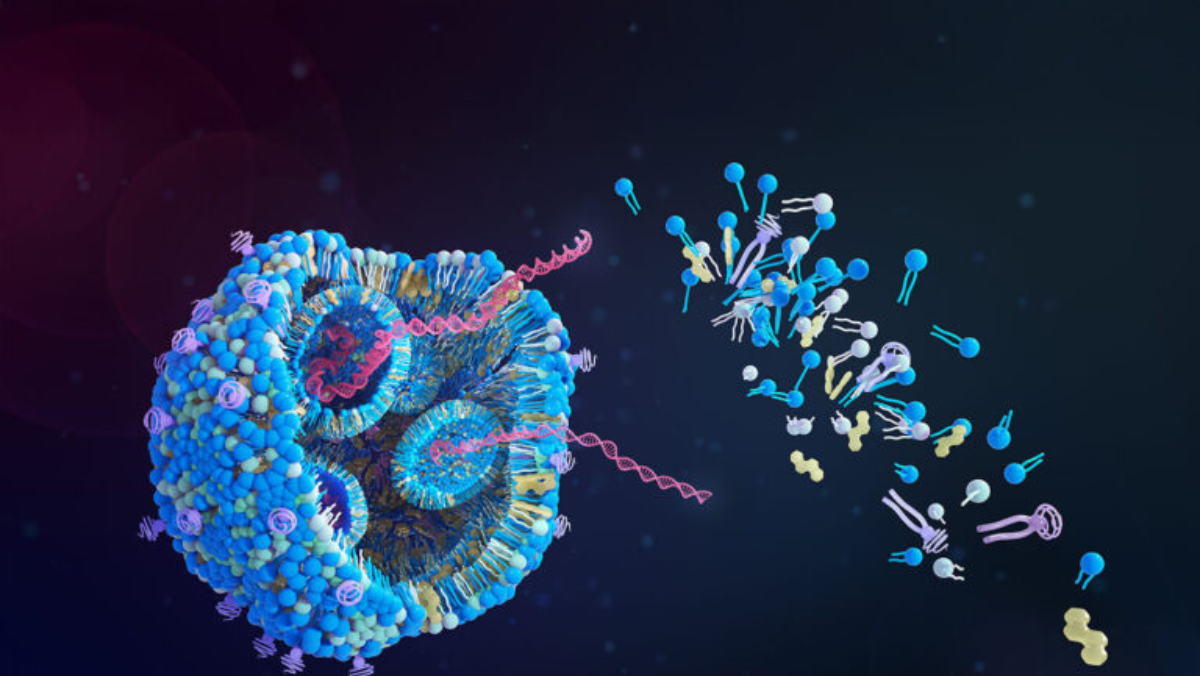
Momentum in mRNA medicine is increasing again, prompting renewed calls for clearer regulation. As the field expands beyond its pandemic role, researchers are applying mRNA technologies to cancer, rare diseases and metabolic conditions. Many argue that existing rules no longer match the pace of scientific work.
The Alliance for mRNA Medicines has become the sector’s main advocate on this issue. Its leaders are urging federal agencies to update the processes governing early research, clinical trials and approvals, warning that outdated systems could slow progress at a moment of rising investment.
Recent industry moves show the scale of that activity. On May 22 2025, Moderna announced plans to expand its US manufacturing network to support faster development of new mRNA therapeutics. Wacker Biotech followed on July 9 with new partnerships to meet growing demand for large-scale production. Replicate Bioscience attracted attention on August 28 with an agreement to work with Novo Nordisk on self-replicating RNA designed to create longer-lasting treatments.
Analysts say these steps reflect a wider shift in drug development, with mRNA platforms now viewed as central technologies rather than niche tools. A policy adviser involved in current discussions said the field risks outgrowing its infrastructure unless regulators offer clearer expectations. Many argue that updated guidance could reduce uncertainty, shorten development timelines and draw in new capital. Sector forecasts point to growth of about 40 per cent in the next few years.
Experts caution, however, that any new rules must balance innovation with safety. Broad frameworks could overlook differences among RNA technologies, while supply-chain constraints and the need for specialised manufacturing skills remain persistent concerns.
Despite these pressures, sentiment in the field is largely optimistic. Researchers say modernised regulation could help convert scientific advances into approved therapies more quickly.
The coming year is seen as important. If Washington moves towards a dedicated regulatory structure, companies could gain a clearer route for the next phase of mRNA development. The combination of scientific progress and commercial activity suggests the US market is entering a potentially significant period for the sector.
Latest News
18 Feb 2026
How the Cloud Powers the Next mRNA Breakthrough16 Feb 2026
Can Europe Win the mRNA Cancer Race?12 Feb 2026
FDA Refusal-to-File Resets mRNA Flu Strategy11 Feb 2026
Britain’s Bet On Better RNA Factories
Related News

TECHNOLOGY
18 Feb 2026
How the Cloud Powers the Next mRNA Breakthrough
INSIGHTS
16 Feb 2026
Can Europe Win the mRNA Cancer Race?
REGULATORY
12 Feb 2026
FDA Refusal-to-File Resets mRNA Flu Strategy
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.